mainmenu
- About Center
- Members
- Research
- Publications
- Facilities
- Super-depth Optical Imaging Lab
- Ultrafast Infrared Spectroscopy Lab
- Ultrafast IR-Visible Spectroscopy Lab
- Instrument and Chemistry Lab
- Molecular Imaging Lab
- Multidimensional Infrared Spectroscopy Lab
- Sample Preparation Lab
- Optical Frequency Comb Spectroscopy Lab
- Single Molecule Imaging Lab
- Spectroscopic Imaging Lab
- Theory and Computation
- Open Access Facility
- Collaborative Research Equipment
- Quantum Spectroscopy Lab
- Ultrafast Raman Probe Spectroscopy Lab
- Equipment Inventory
- Board
Posters and Short Descriptions
Solvation Structure study of Lithium-Ion Battery Electrolytes by FTIR and PFG-NMR spectroscopy
2020 KECS Spring Meeting
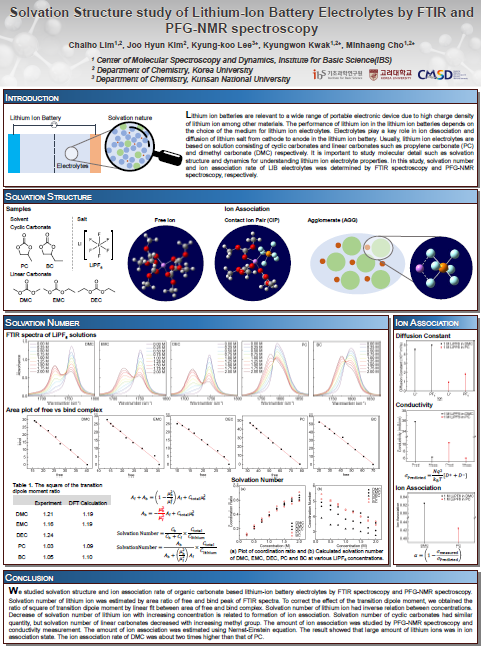
Abstract: Lithium ion batteries are relevant to a wide range of portable electronic device due to high charge density of lithium ion among other materials. The performance of lithium ion in the lithium ion batteries depends on the choice of the medium for lithium ion electrolytes. Electrolytes play a role in ion dissociation and diffusion of lithium salt from cathode to anode in the lithium ion battery. Usually, lithium ion electrolytes are based on solution consisting of cyclic carbonates and linear carbonates such as propylene carbonate (PC) and dimethyl carbonate (DMC) respectively. It is important to study molecular detail such as solvation structure and dynamics for understanding lithium ion electrolyte properties. In this study, solvation number and size were determined by FTIR spectroscopy, PFG-NMR spectroscopy and viscosity measurements. We found solvation number of linear carbonates were larger than that of cyclic carbonates. Amount of ion association were determined by Nernst-Einstein equation based on conductivity measurements.