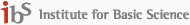IMAGE: (A) SCHEMATIC OF COMPRESSED TIME-REVERSAL MATRIX MICROSCOPE. RANDOM SPECKLE FIELDS GENERATED BY A ROTATING DIFFUSER SEQUENTIALLY ILLUMINATE THE SAMPLE UNDERNEATH AN ABERRATING MEDIUM, AND THE REFLECTED SPECKLE FIELDS ARE MEASURED BY AN OFF-AXIS DIGITAL HOLOGRAPHIC MICROSCOPE. BS: BEAM SPLITTER. OL: OBJECTIVE LENS. (B) EXAMPLES OF MEASURED HOLOGRAM IMAGES FOR SPECKLE FIELDS. (TOP : INTENSITY, BOTTOM : PHASE) view more
CREDIT: INSTITUTE FOR BASIC SCIENCE
Microscopes are an important tool in biomedical research as it allows for detailed observation and imaging of tissues. Since biological materials are opaque by their nature, severe light scattering occurs as light travels through tissues, which induces a high level of background noise and complex optical aberration. Therefore, typical light microscopes mostly allow us to see the surface of the tissues, and details that are multiple cell layers deep are out of reach for many microscopes. This makes taking high-resolution optical images of microstructures deep inside tissues highly challenging.
About one year ago, a research group led by Prof. CHOI Wonshik from the Center for Molecular Spectroscopy and Dynamics (CMSD) within the Institute of Basic Science (IBS) showcased an imaging technique called “reflection matrix microscopy”, which combined the powers of both hardware and computational advanced adaptive optics. In contrast to conventional imaging, it measures a reflection matrix that contains all accessible information about the relationship between the input and output fields of an imaging system, including objects of interest. A clear undistorted image of the object can then be extracted from the measured matrix by post-digital image processing.
As such, the technology emerged as an appropriate candidate for label-free non-invasive high-resolution optical imaging deep inside biological tissues. The matrix imaging certainly outperforms most conventional AOs. For example, the researchers demonstrated that this technology was powerful enough to ‘see through’ an intact mouse skull and allow for accurate imaging of neurons underneath.
Despite its amazing performance, the reflection matrix microscopy was not without drawbacks. Measuring the entire reflection matrix is time-consuming and vulnerable to external perturbations because a large number of interferometric images for all accessible input illumination fields needs to be measured. While a more sparse sampling can speed up the process, insufficient sampling can lead to the limited capability to correct distortions. Therefore, this means that real-time volumetric imaging of living samples was not possible, which led to practical limitations in its application to biodynamic studies.
In the latest research published in the journal Light: Science & Applications, the same IBS group recently unveiled a new and improved version of their previous AO microscope technology. This new real-time volumetric AO imaging system enables 3D imaging over a wide depth range in highly aberrated samples while minimizing image degradation.
To speed up data acquisition, Choi’s team used a compressed sensing technique in the context of matrix imaging. They simply introduced a rotating optical diffuser in their previously deployed reflection matrix microscope to sequentially illuminate unknown speckle patterns on a sample. Then, they obtained a compressed reflection matrix by taking a much smaller number of speckle images than previously required, which reduced the matrix acquisition time by nearly 100 times.
In image post-processing, they employed a compressed “time-reversal matrix” and a unique algorithm to identify sample information and aberrations separately. The advantage of this technique is that it not only dramatically reduces matrix acquisition time but also eliminates the need for careful calibration or specific selection of illumination patterns to be used.
Compressed time-reversal matrix imaging allows for almost real-time volumetric AO imaging. The new microscope’s capabilities were demonstrated by aberration-free 3D imaging of myelin nerve fibers in a mouse brain. The data acquisition time was only 3.58 seconds for volumetric imaging of 128 × 128 × 125 μm3 tissue, with a diffraction-limited lateral resolution of 0.45 μm and an axial resolution of 2 μm.
It is expected to open a new avenue for the practical application of matrix imaging in all fields of wave engineering, including biomedical imaging. Associate Director Choi said, “Faster reflection matrix imaging technology is expected to enable real-time, nondestructive 3D optical diagnosis in the future, which will lead to faster diagnosis and advances in neuroscience research. We will further develop it to broaden the scope of its application in all wave engineering disciplines, including biomedical imaging.”
JOURNAL
Light Science & Applications
METHOD OF RESEARCH
Experimental study
SUBJECT OF RESEARCH
Animals
ARTICLE TITLE
High-throughput volumetric adaptive optical imaging using compressed time-reversal matrix
ARTICLE PUBLICATION DATE
14-Jan-2022