mainmenu
- About Center
- Members
- Research
- Publications
- Facilities
- Super-depth Optical Imaging Lab
- Ultrafast Infrared Spectroscopy Lab
- Ultrafast IR-Visible Spectroscopy Lab
- Instrument and Chemistry Lab
- Molecular Imaging Lab
- Multidimensional Infrared Spectroscopy Lab
- Sample Preparation Lab
- Optical Frequency Comb Spectroscopy Lab
- Single Molecule Imaging Lab
- Spectroscopic Imaging Lab
- Theory and Computation
- Open Access Facility
- Collaborative Research Equipment
- Quantum Spectroscopy Lab
- Ultrafast Raman Probe Spectroscopy Lab
- Equipment Inventory
- Board
Posters and Short Descriptions
Reaction Mechanism study of Cyanide Catalyzed Synthesis of Benzoxazole using Reaction IR Spectroscopy
124th Summer Symposium of KCS-Physical Chemistry Division
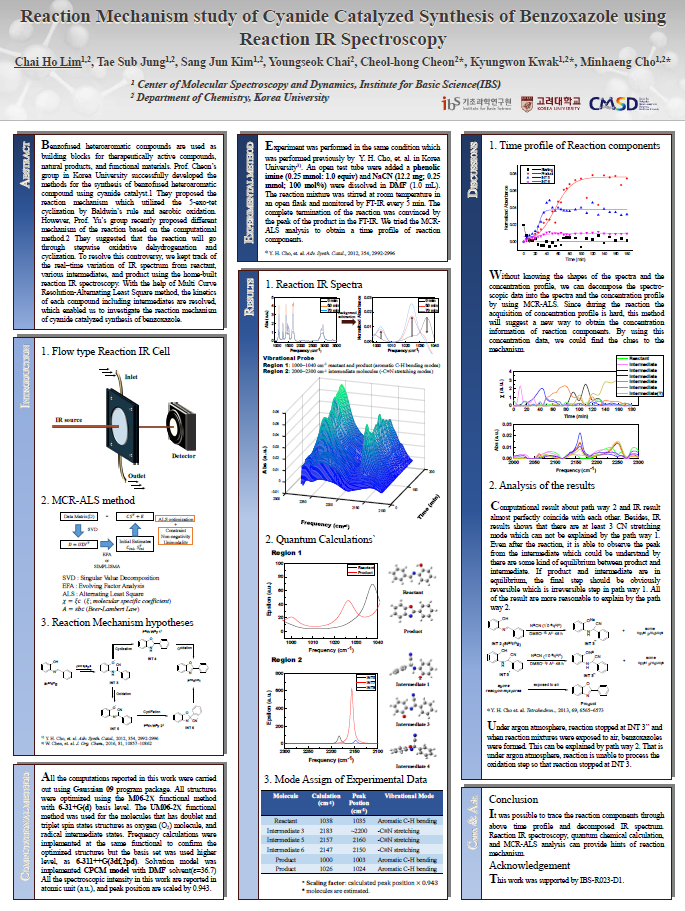
Benzofused heteroaromatic compounds are used as building blocks for therapeutically active compounds, natural products, and functional materials. Prof. Cheon’s group in Korea University successfully developed the methods for the synthesis of benzofused heteroaromatic compound using cyanide catalyst.1 They proposed the reaction mechanism which utilized the 5-exo-tet cyclization by Baldwin’s rule and aerobic oxidation. However, Prof. Yu’s group recently proposed different mechanism of the reaction based on the computational method.2 They suggested that the reaction will go through stepwise oxidative dehydrogenation and cyclization. To resolve this controversy, we kept track of the real–time variation of IR spectrum from reactant, various intermediates, and product using the home-built reaction IR spectroscopy. With the help of Multi Curve Resolution-Alternating Least Square method, the kinetics of each compound including intermediates are resolved, which enabled us to investigate the reaction mechanism of cyanide catalyzed synthesis of benzoxazole.
References
[1] Cho, Y.H.; Lee, C.Y.; Cheon, C.H.; Cyanide as a powerful catalyst for facile synthesis of benzofused heteroaromatic compounds via aerobic oxidation. Tetrahedron. 2013. 69, 6565–6573.
[2] Chen, W.; An, W.; Wang, Y.; Yu, A.; Mechanisms of Metal-Free Aerobic Oxidation to Prepare Benzoxazole Catalyzed by Cyanide: A Direct Cyclization or Stepwise Oxidative Dehydrogenation and Cyclization? J. Org. Chem. 2016.81, 10857–10862.